The EVO Collamer Lens Transends Challenges of Earlier ICLs
Newest FDA approved refractive surgery draws on decades of advances to give patients maximum correction with minimal risk.
Lance Kugler, MD and Drew Dickson, MD
The decades-long evolution of refractive surgery is a story of incremental advances in technique and technology, with each innovation aiming to be more inclusive and less invasive than the last. The most recent refractive device to gain U.S. Food and Drug Administration approval in the US – the EVO Visian (STAAR) intraocular Collamer lens (ICL) – offers major advances in both areas as it treats a wide range of prescriptions without removal of corneal tissue. As investigators in the FDA trial responsible for the EVO ICL’s approval, we witnessed excellent post-op visual outcomes, consistent patient satisfaction, and time-saving efficiencies associated with this lens. Since then, we have gone on to successfully perform EVO surgery on hundreds of patients throughout the post-approval phase.
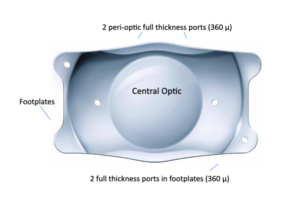
Although this single-piece, posterior chamber phakic refractive IOL only recently became available in the US, it has a long and successful history internationally, where more than 2 million have been implanted over the past 15 years. Many cataract and refractive surgeons are unfamiliar with how these lenses differ from earlier ICL models, and misconceptions remain. Widespread adoption of earlier ICLs was hampered by the risk of anterior subcapsular cataract (ASCs) and pupillary block, among other things. EVO, which is approved for a treatment of 3D to 20D of myopia and 1D to 4D of astigmatism, almost entirely circumvents those barriers to adoption because of its innovative fabrication and design.
In addition to reducing the rate of ASCs and pupillary block compared with earlier ICL models, EVO ICLs achieve high levels of postoperative uncorrected distance visual acuity (UDVA), and refractive predictability and stability. Due to its ability to correct large amounts of myopia and astigmatism, it has often been shown to produce postoperative vision that is better than pre-op vision.4 In the FDA clinical trial, the average postop vision was better than 20/20, and in the more recent toric EVO ICL study, approximately 75% of patients gained at least one line of vision.5
Wow Factor
On an anecdotal level, as investigators in the EVO FDA trial – and since we’ve been making these lenses available to patients at our practice in the post-approval period – we have seen an overwhelmingly positive reaction to EVO with an immediate post-operative wow factor comparable to LASIK. With respect to LASIK, the EVO ICL is often considered an option only for patients who do not fall within the ‘LASIK candidate’ category, but that is outdated thinking. Everything about how we are positioning the EVO ICL has changed as a result of its central port design. Now that the ICL does not require a PI, a major barrier to adoption has been lifted. The additional level of risk associated with the PI and the inconvenience of an extra pre-op appointment with the earlier ICLs made it difficult to offer patients a ‘LASIK-like’ experience. Now that those barriers are gone, the decision tree looks different than it did before EVO. With this shifting paradigm, comes a shift in the patients to whom we are recommending ICL surgery over LASIK.
As with all surgeries, we do not give patients a menu of options and tell them to pick one. We rely on our knowledge and experience, take all of the relevant factors into consideration, and then make a recommendation. LASIK, as well as PRK and SMILE, are excellent procedures, and the EVO ICL is not in competition with them. It’s simply another excellent option. However, when we look at the big picture and consider each patient’s refractive future, there are benefits to offering a procedure that does not remove corneal tissue from the eye. The removability of the EVO ICL, without permanent change to the corneal thickness or curvature, is therefore an appealing feature to both patients and surgeons.
With previous versions of ICLs, we might pause when offering it to young patients because of concerns that they might develop a cataract earlier than they would otherwise. Whereas with EVO available long-term data suggests that the rate of cataract formation is close to zero.1
Risk/Benefit Profile
Patient satisfaction after EVO is as high as anything currently available. It is not unusual for patients to be seeing 20/20 or better just a few hours post-op. This high patient satisfaction is also reflected in a STAAR survey of over 1500 EVO ICL patients, where it was found that 99.4% said they would undergo the procedure again.6
The risk-benefit profile with respect to removing corneal tissue vs inserting an intraocular implant is at the heart of the evolution of refractive surgery, in general, and ICL surgery in particular. While corneal procedures will undoubtedly continue to play an important role in refractive surgery, the option to offer a safe and effective additive procedure to a wide swath of myopes in the form of the EVO ICL, with its improved risk/benefit profile and patient experience, is indeed a game changer.
Lance Kugler, MD is the Founder and CEO, Kugler Vision, Omaha, NE
Contact: [email protected]
Drew Dickson, MD is a refractive surgeon at Kugler Vision
Contact: [email protected]
Financial Disclosure: Neither Dr. Kugler nor Dr. Dickson have a financial interest in STAAR or EVO Visian ICL.
References
- Kamiya K, Shimizu K, Saito A, et al. Comparison of optical quality and intraocular scattering after posterior chamber phakic intraocular lens with and without a central hole (hole ICL and conventional ICL) implantation using the double-pass instrument. PLoS One. 2013;8(6):e66846.
- Schild G, Amon M, Abela-Formanek C, et al. Uveal and capsular biocompatibility of a single-piece, sharp-edged hydrophilic acrylic intraocular lens with collagen (Collamer): 1-year results. J Cataract Refract Surg. 2004;30(6):1254-8.
- Brown DC, Ziemba SL, Collamer IOL FDA Study Group. Collamer intraocular lens: clinical results from the US FDA core study. J Cataract Refract Surg. 2001;27(6):833-40.
- Kamiya K, Shimizu K, Igarashi A, et al. Comparison of Collamer toric implantable [corrected] contact lens implantation and wavefront-guided laser in situ keratomileusis for high myopic astigmatism. J Cataract Refract Surg. 2008;34(10):1687-1693.
- STAAR Visian Toric ICL post-approval study (TICL-PAS). ClinicalTrials.gov. Updated August 20, 2020. Accessed August, 11, 2022. https://clinicaltrials.gov/ct2/show/NCT04516772.
- Patient Survey, STAAR Surgical ICL Data Registry, 2018.
Image 1: EVO ICL 360µm central port eliminates the need for a preop peripheral iridotomy (PI). The design facilitates the flow of aqueous humor through the lens and allows passive OVD removal, with no need for direct irrigation/aspiration.