Spotlight on CEQUA®
This Spotlight Series article is editorially independent content.
Dry eye disease (DED) is the most common ocular surface disorder, characterized by chronic inflammation.1 Prescription treatment options focus on addressing the inflammatory pathways or excessive tear film evaporation to break the vicious cycle of DED and prevent chronic disease and progression.2
CEQUA®: A Unique Formulation of Cyclosporine
Several therapeutic strategies are currently available for DED therapy, including CEQUA (cyclosporine ophthalmic solution; Sun Pharmaceuticals) 0.09%. CEQUA is a calcineurin inhibitor immunosuppressant that received FDA approval in 2018 and is indicated to increase tear production in patients with keratoconjunctivitis sicca (dry eye). It is the first and only DED treatment to combine cyclosporine A with nanomicellar NCELL technology, which penetrates the aqueous layer of the tear film, resulting in potentially improved ocular tissue penetration.3
Efficacy Data
CEQUA has been studied in multiple clinical trials, demonstrating sustained improvement in DED signs and symptoms.
Phase 4 Study
In a recent single-arm, 12-week, phase 4, multicenter study, CEQUA showed significant improvement in both corneal fluorescein staining (CFS) and modified Symptom Assessment in Dry Eye (mSANDE) scores for patients whose DED was inadequately controlled (ie, still symptomatic and/or exhibiting disease signs) on RESTASIS® (cyclosporine ophthalmic emulsion) 0.05% therapy for at least 3 months.4,5
The study included 124 patients who were administered 1 drop in each eye twice daily for 12 weeks. Assessments for CFS and mSANDE scores were conducted at baseline and at weeks 4, 8 and 12, or upon early discontinuation from the study.4,5
The findings showed statistically significant improvements in CFS and mSANDE scores in as early as 4 weeks of treatment and continued through week 12. The mean total CFS score improved baseline to week 12, as did the mean mSANDE score (P<.0001 for both); see FIGURES.
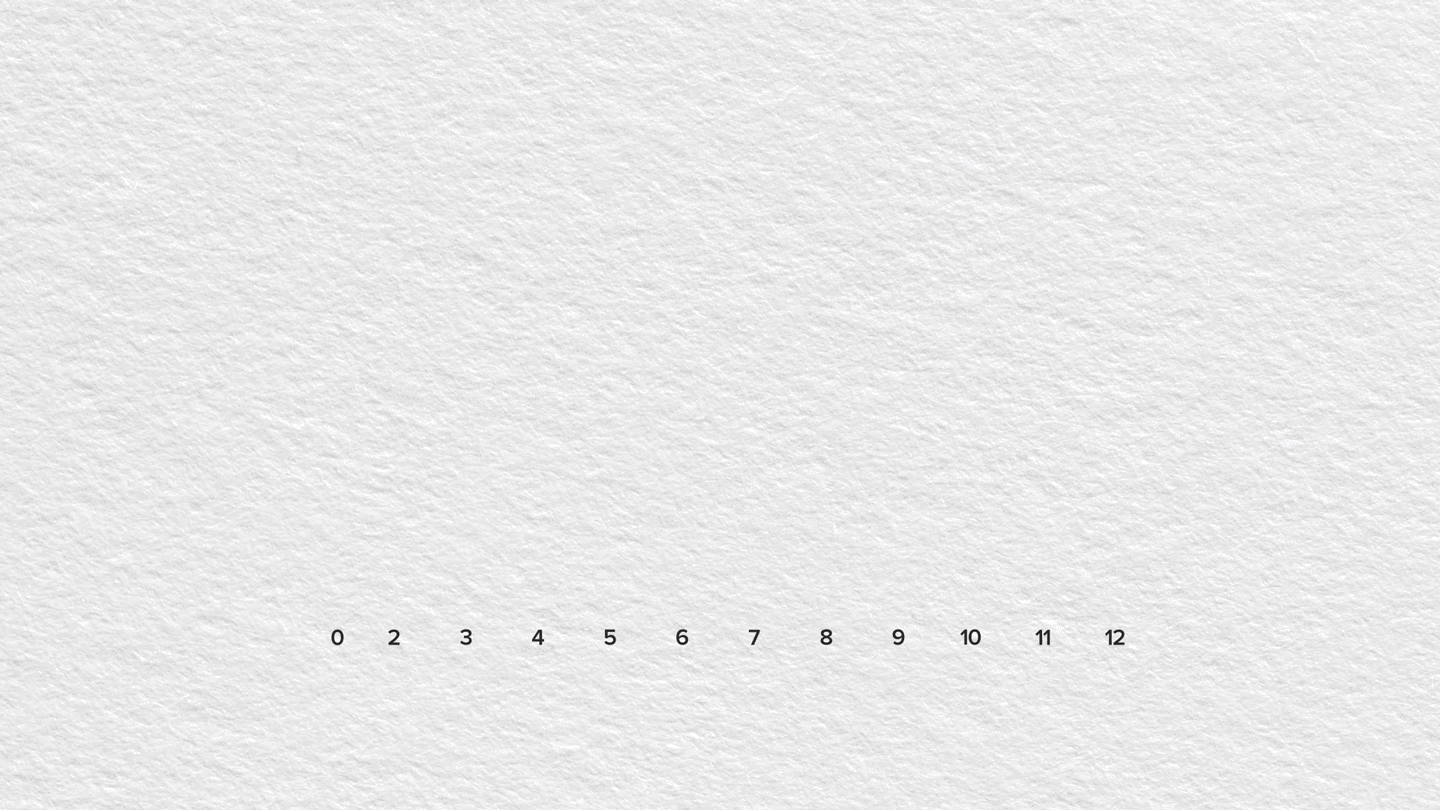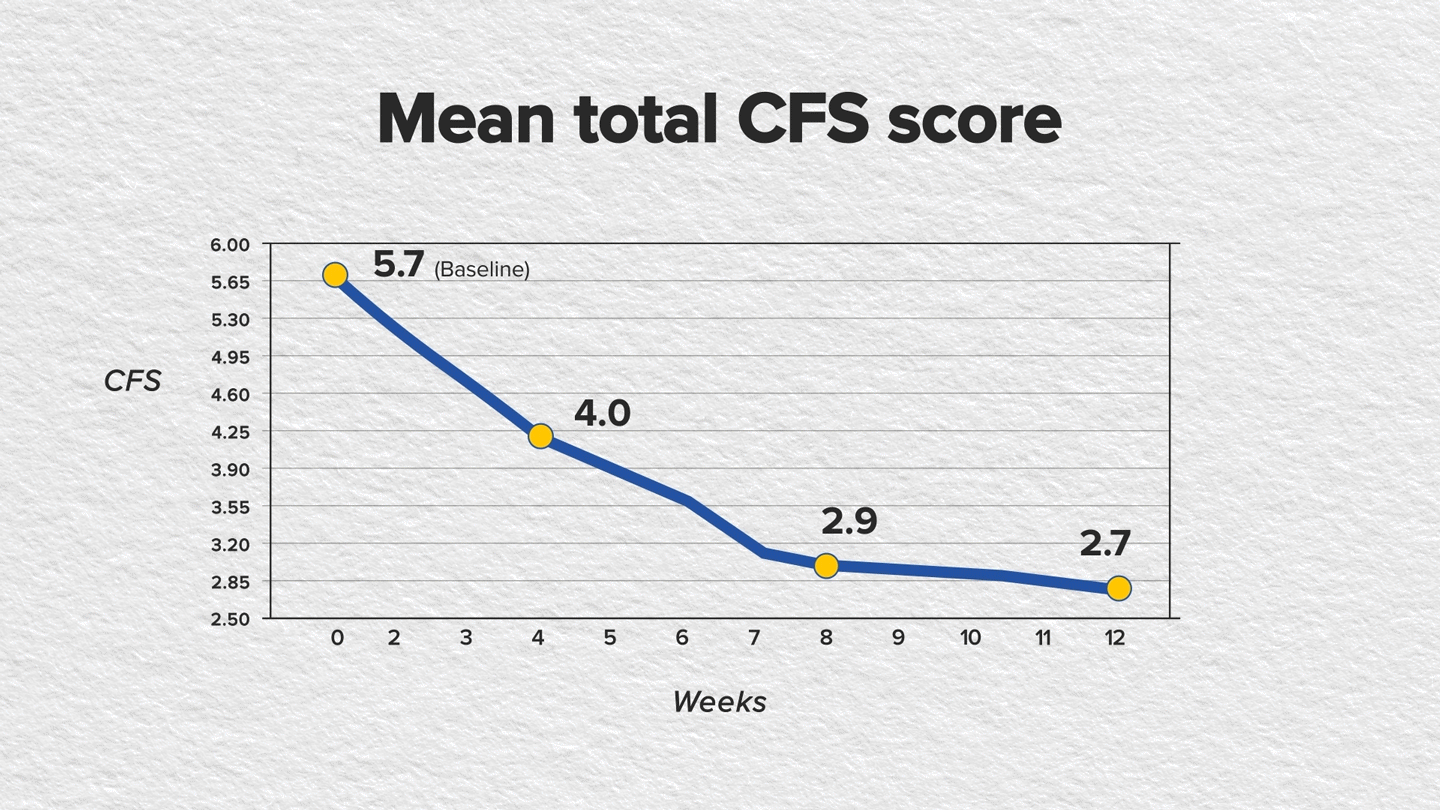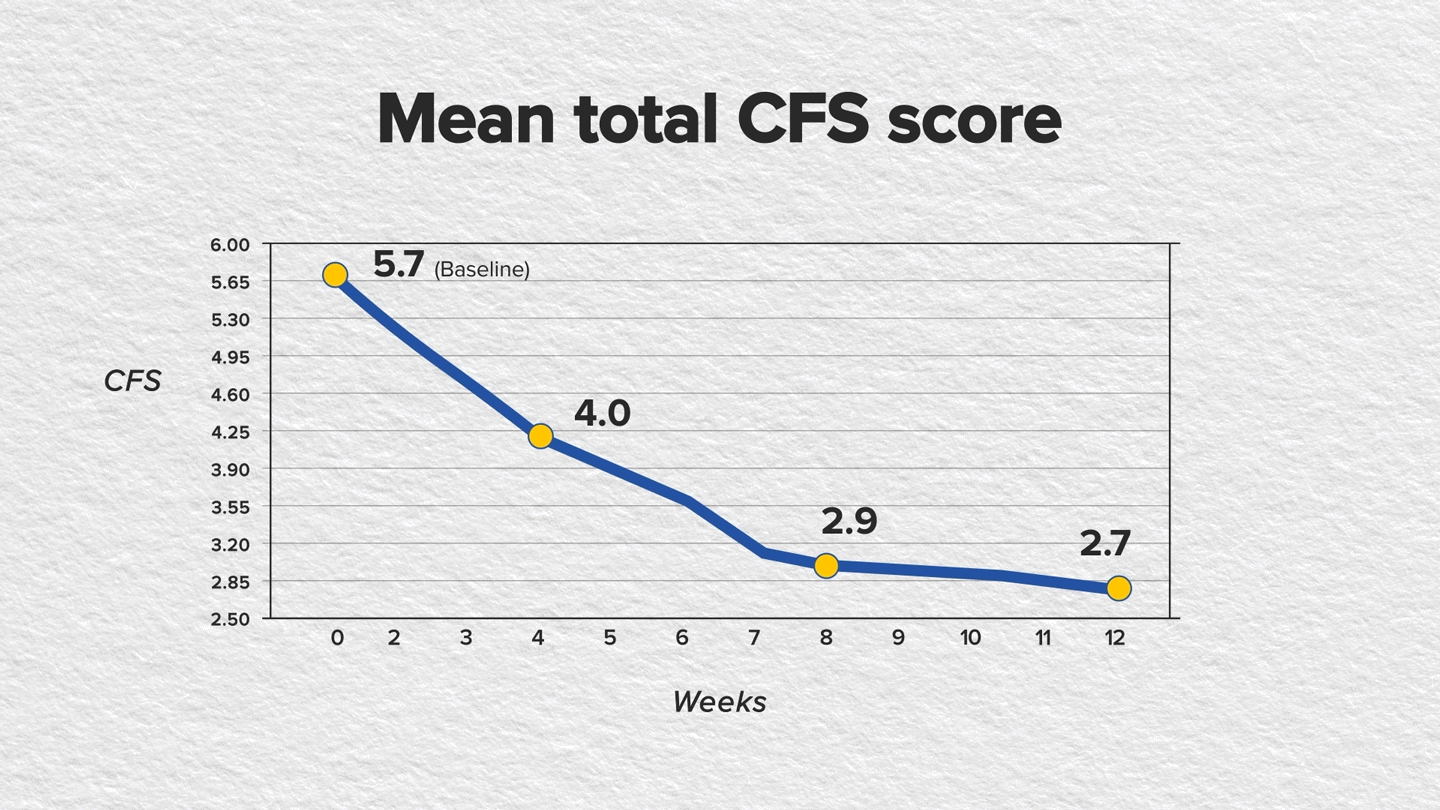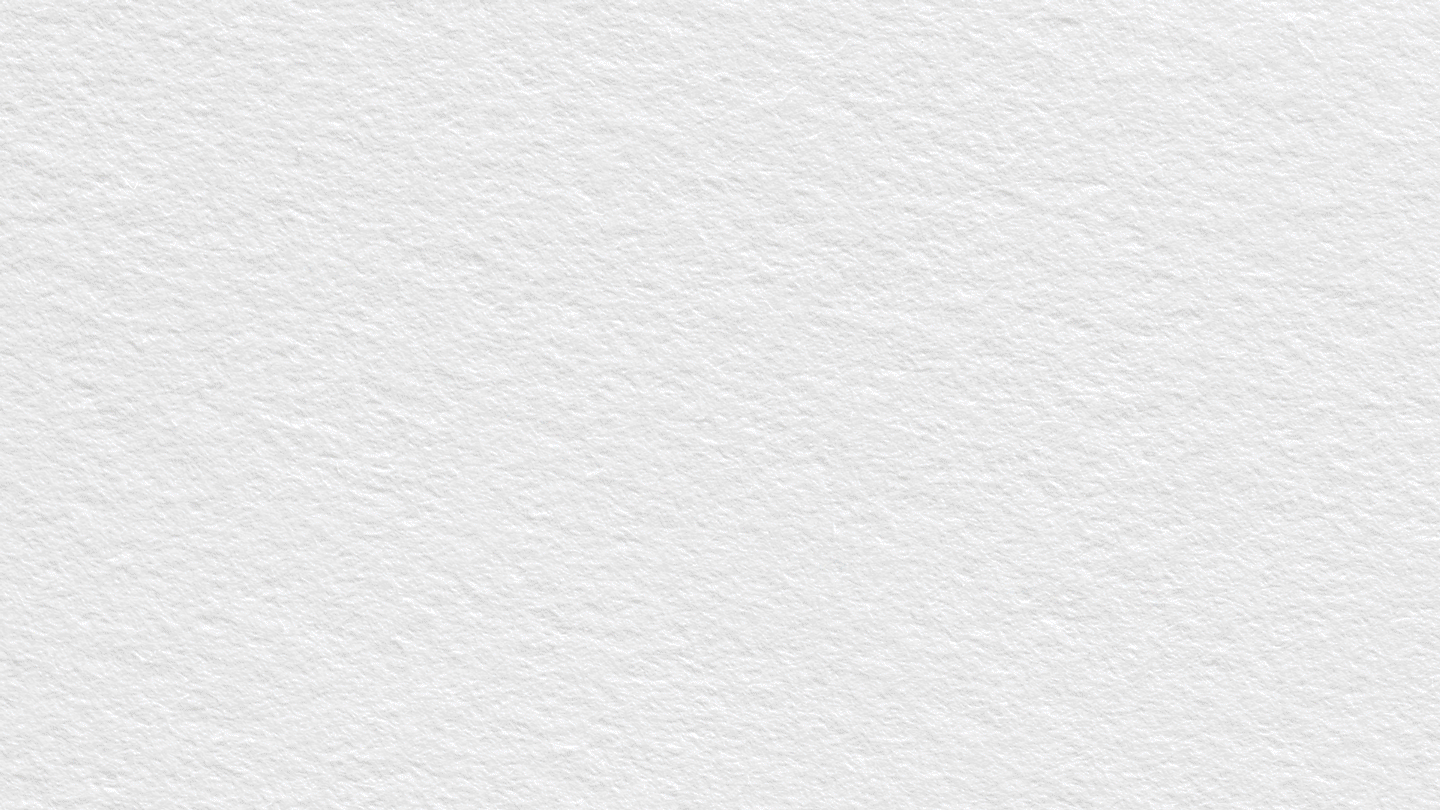
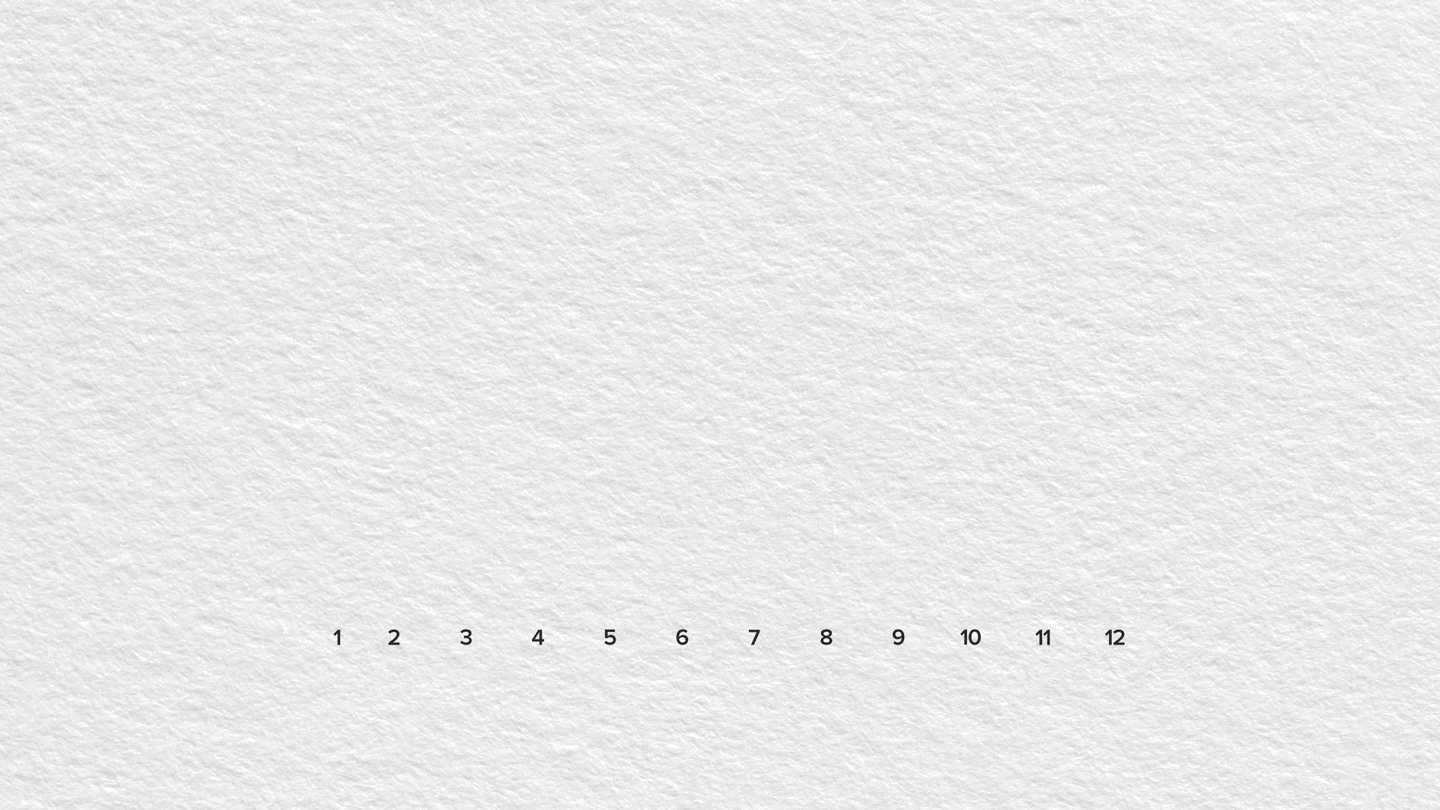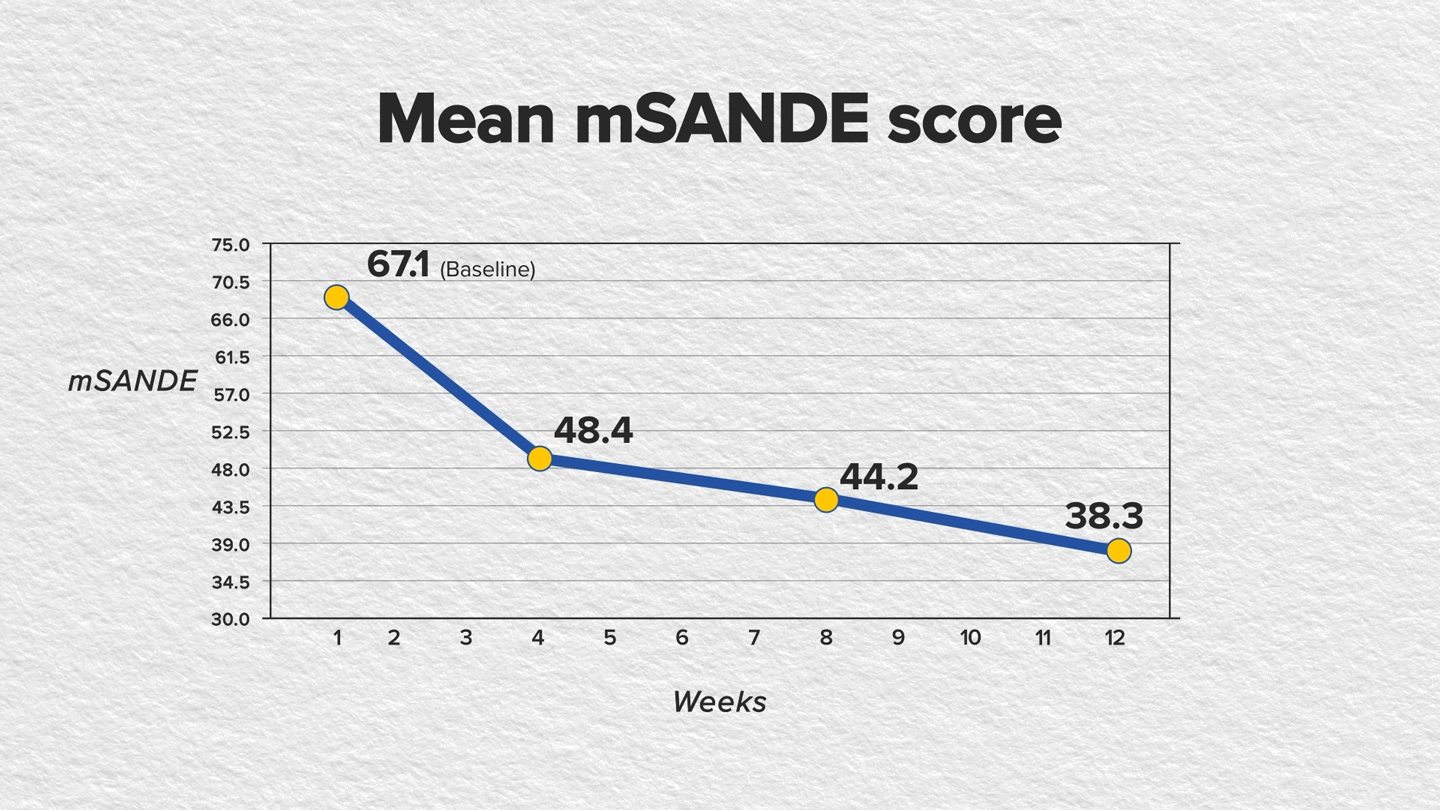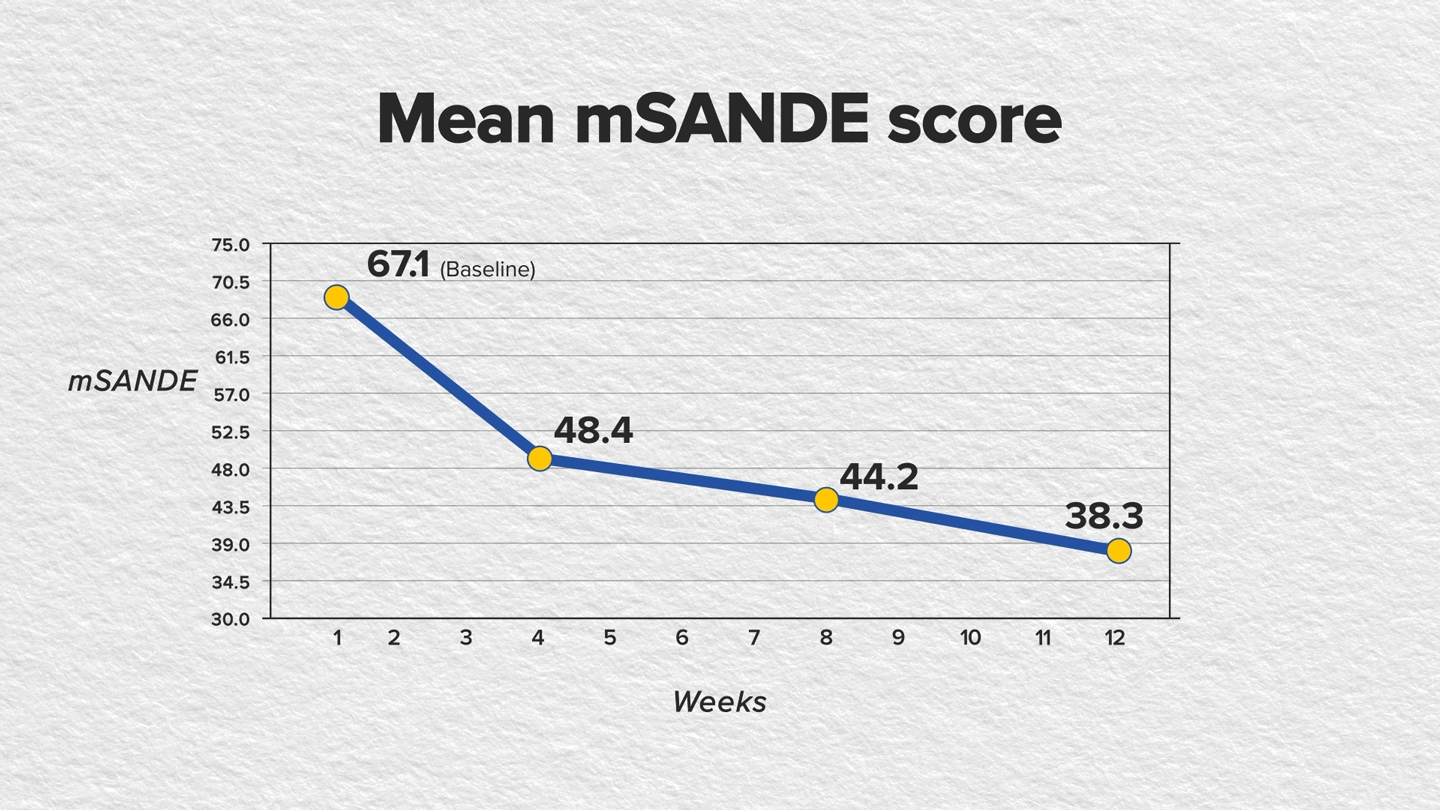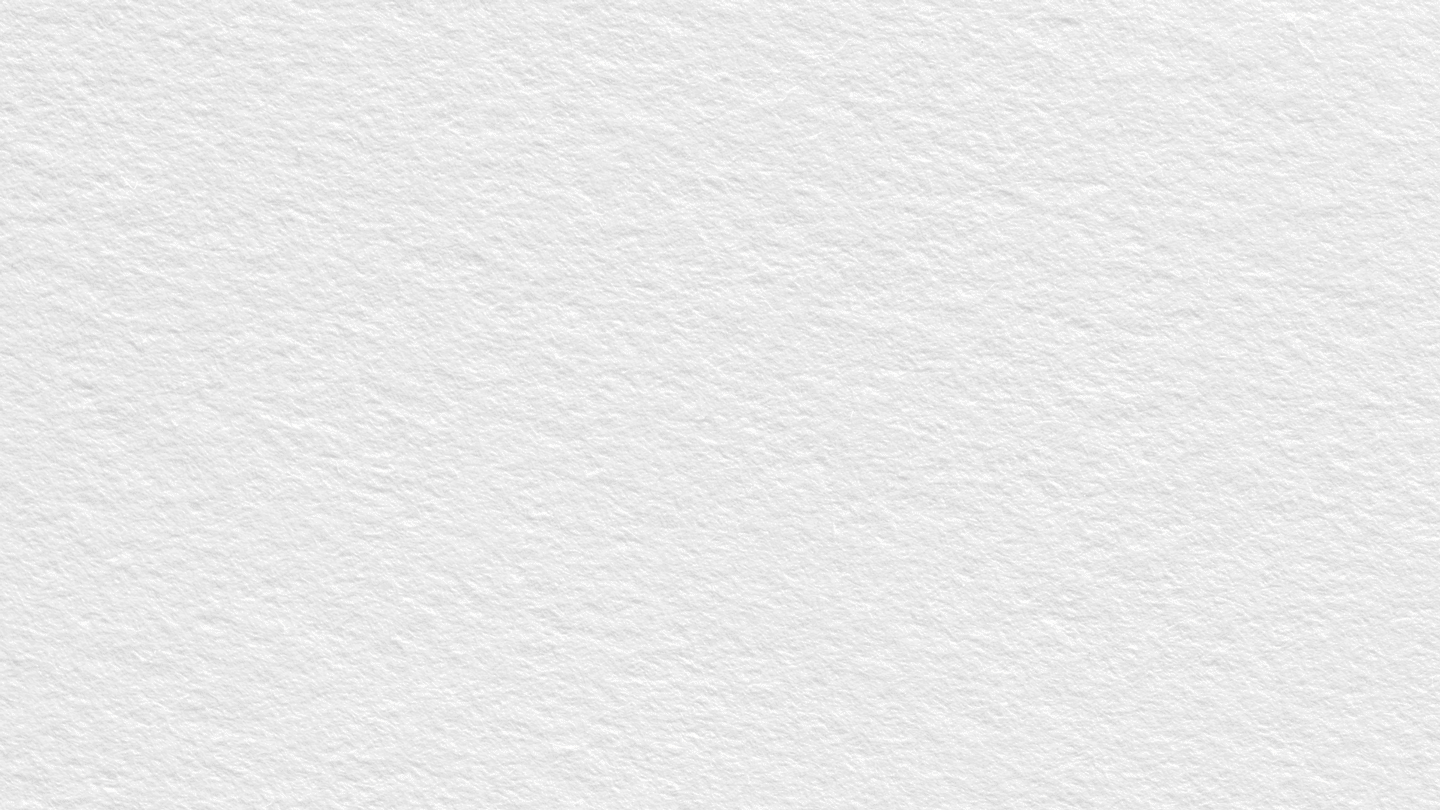
Furthermore, artificial tear use was reduced from 3 times to 1 time per day after switching from RESTASIS to CEQUA.4,5
Efficacy Studies
CEQUA also demonstrated a clinically meaningful improvement in tear production (≥10-mm change from baseline in Schirmer test score) at 3 months compared with vehicle (P<.01 for both) across two 12-week, randomized, vehicle-controlled studies (see FIGURES).

Percentage of patients experiencing a ≥10-mm increase in tear production at day 84

Percentage of patients experiencing a ≥10-mm increase in tear production at day 84
Study 1 included 455 patients (152 received CEQUA) and study 2 included 744 patients (371 received CEQUA).6-8 Additionally, at 3 months in study 2, 65% of central corneas were completely clear versus 56.9% for vehicle (P=.02). At baseline, 38.3% of patients taking CEQUA had complete clearing versus 37.5% with vehicle.8
Safety and Tolerability
CEQUA was generally well-tolerated in clinical trials. The most common adverse reactions reported in >5% of patients were pain on instillation of drops (22%) and conjunctival hyperemia (6%). Other adverse reactions reported in 1% to 5% of patients were blepharitis, eye irritation, headache, and urinary tract infection.6 Consistent with the established safety profile in clinical trials, there were no new safety signals in the phase 4 study. Overall, 58 patients (43.3%) reported at least 1 treatment-emergent adverse event, although most (73.8%) were mild.4,5
Dosing
CEQUA is available in single-use vials that should be discarded immediately after each use. The recommended dosage is 1 drop twice daily (approximately 12 hours apart) into each eye. CEQUA can be used concomitantly with artificial tears, allowing a 15-minute interval between products. Contact lenses should be removed before using CEQUA and may be reinserted 15 minutes following administration.6
For more information, visit https://cequapro.com.
References
- Ouyang W, Yan D, Hu J, et al. Multifaceted mitochondrial as a novel therapeutic target in dry eye: insights and interventions. Cell Death Discov. 2024;10(1):398. doi:10.1038/s41420-024-02159-0.
- Periman LM, Perez VL, Saban DR, et al. The immunological basis of dry eye disease and current topical treatment options. J Ocul Pharmacol Ther. 2020;36(3):137-146. doi:10.1089/jop.2019.0060
- Sun Pharma announces U.S. FDA approval of CEQUA to treat dry eye disease. News release. Sun Pharmaceutical Industries Ltd. August 16, 2018. Accessed January 5, 2025. https://www.sunophthalmics.com/news
- Johnston J. Effect of OTX-101 0.09% on corneal staining and SANDE scores in patients with dry eye disease uncontrolled on cyclosporine ophthalmic emulsion 0.05%. Abstract presented at: American Academy of Optometry 2023, October 12, 2023; New Orleans, LA.
- CEQUA® (cyclosporine ophthalmic solution) 0.09% phase 4 data showed sustained improvement in dry eye disease signs and symptoms in patients switched from Restasis® (cyclosporine ophthalmic emulsion) 0.05%. News release. Sun Pharmaceutical Industries Ltd. October 12, 2023. Accessed January 5, 2025. https://sunpharma.com/wp-content/uploads/2023/10/Press-Release-CEQUA-showed-sustained-improvement-in-dry-eye-disease-patients-switched-from-Restasis.pdf
- Package insert. Sun Pharmaceutical Industries, Inc; 2022.
- Tauber J, Schechter BA, Bacharach J, et al. A phase II/III, randomized, double-masked, vehicle-controlled, dose-ranging study of the safety and efficacy of OTX-101 in the treatment of dry eye disease. Clin Ophthalmol. 2018;12:1921-1929. doi:10.2147/OPTH.S17506
- Goldberg DF, Malhotra RP, Schechter BA, Justice A, Weiss SL, Sheppard JD. A phase 3, randomized, double-masked study of OTX-101 ophthalmic solution 0.09% in the treatment of dry eye disease. Ophthalmology. 2019;126(9):1230-1237. doi:10.1016/j.ophtha.2019.03.050

